A Novel Mechanism of Histone Deacetylase Inhibitor-induced Apoptosis
Source:
Time: 2011-05-12
Histone acetylation is an important epigenetic mechanism controling gene expression. Increased histone acetylation is generally associated with a higher gene transcription activity. Aberrant histone acetylation has been found to be implicated in various pathological processes. Histone deacetylase (HDAC) are significant histone acetylation modification factors, which is overexpressed in a variety of cancer tissues. HDAC inhibitors are potent apoptosis inducers to many type of cancer cells, and they have no obvious toxic effect in untransformed cells. Therefore, HDAC inhibitors are a class of promising anti-cancer reagents.
Embryonic carcinoma cells are a group of poorly differentiated and highly aggressive cells in teratocarcinoma tissue. They are not sensitive to classical chemotherapeutic agent treatment and are closely associated with the relapse and metastasis of teratocarcinoma. It has been reported that HDAC inhibitors are able to induce apoptosis in embryonic carcinoma cells. However, the underlying mechanism remains to be identified.
SHU Guangwen and his colleagues in Dr. SONG Jianguo’s lab, revealed that Zac1 upregulation induced by HDAC inhibitors plays a major role in HDAC inhibitor-induced embryonic carcinoma cell apoptosis.
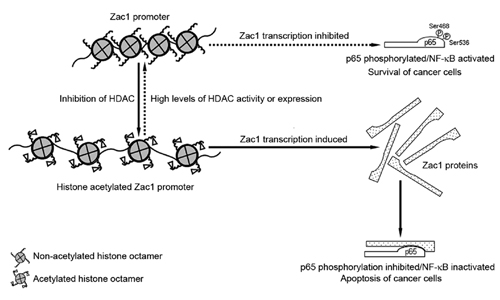
Their results demonstrated that Zac1 functions as an NF-κB suppressor. Zac1 upregulation is mediated by histone acetylation of the Zac1 promoter region. Zac1 bind the C-terminus of the NF-κB p65 subunit and suppresses the phosphorylation of p65 at Ser468 and Ser536 residues, which inhibited the NF-κB activity, leading to the apoptosis of embryonic carcinoma cells. The presented study provides a novel understanding on the mechanism underlying HDAC inhibitor-induced apoptosis, which can also be significant for developing the molecular targets in the treatment of tumors.
This work entitled “Zac1 is a histone acetylation-regulated NF-κB suppressor that mediates histone deacetylase inhibitor-induced apoptosis” was published online in Cell Death and Differentiation on May 6th, 2011. The work is carried out in collaboration with Dr. WANG Chen (SIBCB), and was supported by the National Natural Science Foundation of China.
AUTHOR CONTACT:
SONG Jianguo
Institute of Biochemistry and Cell Biology
Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences
Shanghai, China 200031
Summary by a schematic illustration
The histone of Zac1 promoter in untreated embryonic carcinoma cells was hypoacetylated, which repressed Zac1 gene transcription. HDAC inhibitors induced histone acetylation and increased Zac1 expression. Zac1 protein bound the NF-κB p65 subunit and inhibited its phosphorylation at Ser468 and Ser536, leading to the suppression of NF-κB activity and the induction of cell apoptosis. The reverse process as indicated by a dashed arrow shows a potential pathway that maintains NF-κB activity and is important for cancer cell survival. High levels of HDAC or HDAC activity kept the Zac1 promoter in hypoacetylation status and thus suppressed Zac1 expression, which accounted for the relatively high phosphorylation levels at Ser468 and Ser536 residues of p65 protein and the relative high NF-κB activity.
(Image provided by Dr. SONG Jianguo)
 Appendix:
Appendix: