Scientists Define Pathophysiologic Changes of FOXP3 at the Atomic Level
Source:
Time: 2012-06-28
Why do genetic mutations of the forkhead box P3 (FOXP3) protein cause autoimmunity, infectious diseases and cancers? New studies have uncovered regulatory structural features of the FOXP3 complex, which explain certain disease-causing mutations by definable physical effects on FOXP3 dimer conformation.
FOXP3 is a master transcriptional factor of regulatory T cells that mediates multiple essential roles in controlling immune responses against pathogens. Mutations of FOXP3 leads to a fatal recessive immune disorder called “IPEX (Immunodysregulation, polyendocrinopathy and enteropathy, X-linked)”. Other mutations or abnormal functions of FOXP3 are implicated in breast cancer and virus infection. FOXP3 has intrigued biologists to establish a mechanistic model that correlates FOXP3 three-dimensional structure with its biological functions.
Through a collaboration with American researchers, ZHOU Zhaocai’s group from Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences, has recently dissected the atomic features of the dimeric assembly of the FOXP3 coiled coil region, taking a crucial step towards understanding its regulatory nature.
After several years of complex and detailed research, they found that FOXP3 molecules assume an antiparallel dimeric conformation which is critical for DNA binding and targeted gene suppression or activation. It is now clear that, in response to discrete celluar signals, posttranslational modifications such as lysine acetylation may regulate FOXP3 functions by modulating its inter-subunit bonding. In contrast, disease-causing mutations disrupt inter-subunit bonding of the FOXP3 dimer, depriving the complex of regulatory capacity.
Their work entitled “Structural and biological features of FOXP3 dimerization relevant to regulatory T cell function”, which was funded by the 973 program of the Ministry of Science and Technology of China and the National Natural Science Foundation of China, has been published on June 28th in the journal of Cell Reports.
AUTHOR CONTACT:
ZHOU Zhaocai
Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences, Shanghai, China
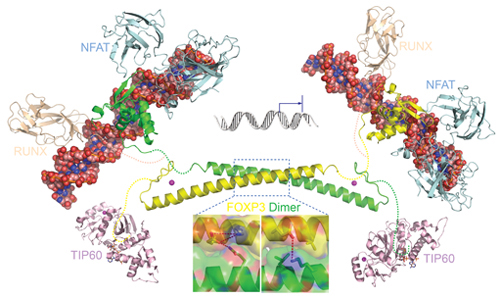
Model of the FOXP3 regulatory complex
The N-terminal domain of FOXP3 molecule may recruit TIP60 and HDAC7 to form a repressor unit, while the forkhead domain functions as a DNA binding unit. To regulate gene transcription, the repressor unit from one FOXP3 molecule cooperates with the DNA binding unit from another FOXP3 molecule. In this process, acetylation mediated modulation of the FOXP3 coiled coil plays a critical role, while disease-causing mutations disrupt inter-subunit bonding and impaired the regulatory capacity of the FOXP3 anti-parallel dimer.
 Appendix:
Appendix: