New Study Provides Insight into the Accurate Recognition of tRNA by Leucyl-tRNA Synthetase
Source:
Time: 2012-12-28
Aminoacyl-tRNA synthetases (aaRSs) are an old protein family among all cellular organisms. Recently researchers from Chinese Academy of Sciences revealed the structural importance of the C-terminal Domain in modulating leucyl-tRNA synthetase (LeuRS)-tRNA interaction in aminoacylation and editing processes using Mycobacterium tuberculosis LeuRS (MtbLeuRS) as the model system.
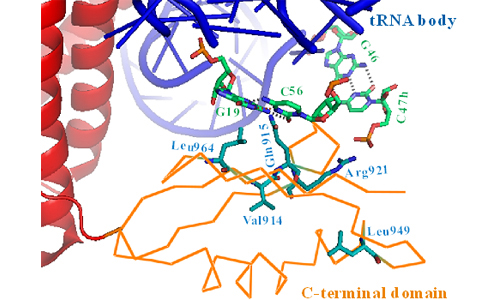
Ms. HU Qinghua and her colleges led by Professor WANG Enduo from Shanghai Institute of Biochemistry and Cell Biology, CAS established the first effective MtbLeuRS system with high activity and quantitatively analyzed its editing properties. Further mutagenesis identified several crucial residues in the MtbLeuRS CTD that contribute in tRNA binding capacities as suggested by fluorescence quenching assays and yeast three-hybrid studies. They also proposed the flexibility of the CTD facilitates the conformational rearrangement in the processes of substrate binding, aminoacylation and editing.
This study extends our knowledge on the accurate recognition of tRNA by LeuRS and provides a platform for the development of novel antitubercular drugs targeting MtbLeuRS.
This study entitled “Crucial role of the C-terminal domain of Mycobacterium tuberculosis leucyl-tRNA synthetase in aminoacylation and editing” was published online in Nucleic Acids Research on December 24th, 2012. It was funded by the National Natural Science Foundation of China, Ministry of Science and Technology and Chinese Academy of Sciences.
AUTHOR CONTACT:
WANG Enduo
Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences, Shanghai, China
Relative orientation of the CTD relative to the tRNA in TtLeuRS-tRNALeu structure (Image provided by Prof. WANG Enduo).
 Appendix:
Appendix: