Innate immunity is the first line of defense against pathogens. After detecting pathogen-associated molecular patterns, Toll-like receptors (TLRs) initiate innate immune response via activating the adaptor molecule TRAF6 to remove pathogens. However, excessive or prolonged inflammatory responses can cause severe host damage or even death; meanwhile chronic inflammation could lead to the development of tumors. Thus the balance between inflammation and tolerance must be fine maintained.
TLR-TRAF6 signaling has been extensively investigated due to its critical roles in inflammation and cancer. Yet increasing studies suggest a picture of TLR-TRAF6 pathways far more complicated than previously thought. Now, a research group led by Prof. ZHOU Zhaocai from the Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences (CAS) identified the kinase MST4 as a “brake” molecule that dynamically responds to inflammatory stimuli. By analyzing clinical samples of sepsis and malaria, researchers found that MST4 expression was dysregulated in these infectious diseases. MST4 expression displayed a dramatic fluctuation upon inflammatory stimulation, and such kinetics is correlated with inhibition of inflammatory cytokine production. Mechanistically, MST4 directly interacts with and phosphorylates TRAF6 to restrict its auto-ubiquitination and signaling activity. Thus MST4 acts as a “brake” on TLR-TRAF6-mediated inflammatory responses. Analyses in mouse model of sepsis confirmed these findings, and further revealed that MST4 protects host from septic shock largely through macrophage. This study uncovers new mechanism in controlling innate immunity, and it may also provide new clues to the pathogenesis of some inflammation-related diseases including cancer.
This work entitled “The kinase MST4 limits inflammatory responses through direct phosphorylation of the adaptor molecule TRAF6” was published online in Nature Immunology on Feb 2nd, 2015.
This work was funded by the National Natural Science Foundation of China, the Ministry of Science and Technology of China, the CAS and the Science & Technology Commission of Shanghai Municipality.
CONTACT:
ZHOU Zhaocai, Ph.D, Principal Investigator
Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences
Shanghai 200031, China.
Email: zczhou@sibcb.ac.cn
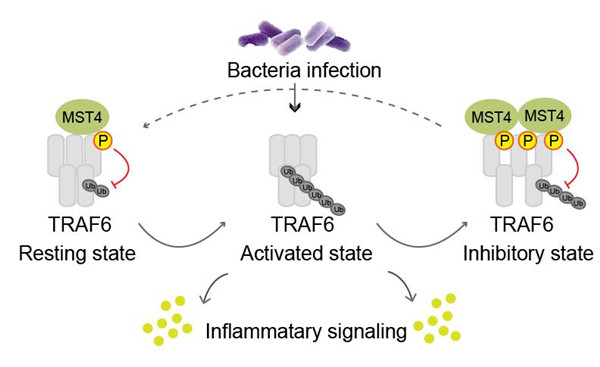
Fig. MST4 limits inflammatory response via inhibition of TLR-TRAF6 signaling. (Image provided by Prof. ZHOU Zhaocai)
 Appendix:
Appendix: