Aging is a common and conserved phenomenon among all the living organisms. Genome instability has long been implicated as a major cause of aging. Telomeres, the physical ends of eukaryotic linear chromosomes, are maintained by telomerase in most cases. However, due to the resemblance of telomeres to DNA double strand breaks (DSBs), homologous recombination can not be eliminated from telomeres even in the presence of telomerase. Recombination activities at telomeres may elicit genome instability and act as a threat to cellular lifespan.
Recently, Prof. ZHOU Jinqiu and his group at the Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, CAS, identified a telomere recombination regulator, the yeast KEOPS subunit Cgi121, as a novel longevity regulator. They provided evidence that inhibition of telomere recombination by inactivation of Cgi121 significantly extends lifespan.
Previous work by ZHOU group showed that activation of telomere recombination by depletion of telomerase accelerates cellular aging, suggesting a dark side of telomere recombination on longevity(PLoS Genet2009, 5:e1000535.). Later, they performed a genetic screening for telomere recombination regulators and identified several genes required for telomere recombination, including the evolutionarily conserved KEOPS complex(PLoS Genet2013, 9:e1003208.). In this study, PENG Jing, a postdoctoral fellow in Dr. ZHOU lab, and the colleagues confirmed that telomere recombination accelerates aging and inhibition of telomeric recombination restores lifespan. In addition, they found that Cgi121 is specifically required for telomere recombination. Deletion of CGI121 gene compromises telomere recombination efficiency, and strikingly extends replicative lifespan. Notably, Cgi121 does not affect recombination at another highly recombinogenic region, the rDNA region. Inhibition of recombination at telomeres and the rDNA locus causes additive effects on slowing down the aging process. Further mechanistic characterization revealed that Cgi121 may affect aging by its function in the generation of telomeric single-strand DNA to promote telomere recombination. This study suggests that recombination activities at telomeres interfere with telomerase to pose a negative effect on cellular longevity.
A paper entitled “Inhibition of Telomere Recombination by Inactivation of KEOPS Subunit Cgi121 Promotes Cell Longevity” was published online in PLOS Genetics on March 30th, 2015. This study was supported by the grants from the National Natural Science Foundation of China and the Ministry of Science and Technology of China.
AUTHOR CONTACT:
ZHOU Jinqiu, Principal Investigator
Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences
Shanghai 200031, China
Email: jqzhou@sibcb.ac.cn
Phone: +86-21-54921076
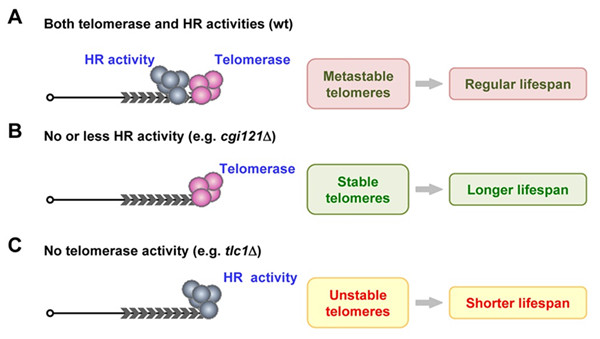
Fig. Recombination and telomerase activities competitively function at telomeres to affect cellular lifespan.
(A) In wild-type cells, telomerase and HR competes at telomeres to affect cell longevity. (B) In cells that have no or less HR activity (e.g. cgi121Δ mutant), telomerase is not disturbed by HR activity. Telomeres are more stable, and results in extended lifespan. (C) In telomerase-deficient cells (e.g. tlc1Δ cells), HR activity is the sole force to maintain telomeres, leading to unstable telomeres and shortened lifespan.(Image provided by Prof. ZHOU Jinqiu`s group)
 Appendix:
Appendix: