Cardiolipins (CLs) are featured lipids of the inner membrane of mitochondria (MIM) and are essential for its proper function. The majority of CLs are synthesized at the MIM using phosphatidic acids (PA) as precursors. In the cell, PA is mainly produced at the ER membrane, then transported from the ER membrane to the outer membrane of mitochondria (MOM) and further to the MIM. The transport of PA from MOM to MIM is mediated by the MSF1-PRELI family protein complex Ups1-Mdm35, and feedback inhibited by CL; however, the underlying molecular mechanism remains unclear.
Recently, researchers from groups of Professor DING Jianping (Institute of Biochemistry and Cell Biology) and Professors ZHANG Peng and XUE Hongwei (Institute of Plant Physiology and Ecology), Shanghai Institutes for Biological Sciences, CAS jointly reported the Ups1-Mdm35-PA complex structure and the structural-based functional analyses which reveal the molecular basis of Ups1-Mdm35 mediated intramitochondrial PA transport.
The structure of Ups1-Mdm35 complexed with PA was determined at 2.0 Å resolution. In the structure, Ups1 features an antiparallel β-sheet barrel plus three α-helices while Mdm35 adopts a three-helical clamp-like structure to wrap around Ups1 to form a stable complex. An inside pocket was identified in Ups1 to accommodate PA, and the pocket is coved by a lid formed by α2 helix. Structural based functional analyses show that the hydrophobic residues lining the pocket and α2 helix are critical for PA binding and transfer. In addition, a hydrophilic patch on the surface of Ups1 near the PA phosphate-binding site also plays an important role in the function of Ups1-Mdm35. Based on the Ups1-Mdm35-PA structure, a structural model of Ups1-Mdm35 bound with CL was built, which suggested the mechanism of the feedback inhibition of Ups1-Mdm35 mediated PA transport by CL. Ups1-Mdm35 represents the first structure of MSF1-PRELI family proteins involving lipid/PA trafficking, and shed new lights on the mechanism of intra-mitochondrial lipid transport.
This research entitled “Structural basis of intramitochondrial phosphatidic acid transport mediated by Ups1-Mdm35 complex” has been published in EMBO reports on Jun 13, 2015. The work was supported by grants from the National Natural Science Foundation of China and Chinese Academy of Sciences.
CONTACT:
DING Jianping, Principal Investigator
Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences
Shanghai 200031, P. R. China.
Email: jpding@sibcb.ac.cn
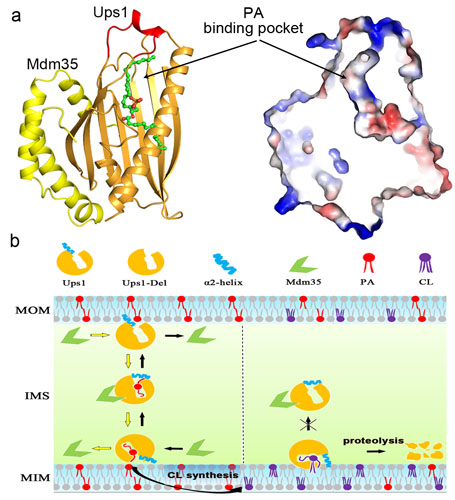
Figure Structural model of Ups1-Mdm35 mediated intra-mitochondrial PA transport and feedback inhibition. (a) Structure of the Ups1-Mdm35-PA complex shows the PA binding pocket inside Ups1. (b)Working model of Ups1-Mdm35 mediated intra-mitochondrial PA transport: Ups1 attach to the MOM via charge interactions; Mdm35 wrap around Ups1 to form stable complex; depending on the different concentration of PA at MOM and MIM, α2 lid of the pocket undergoes conformational change to bind or release PA; enough CL at MIM fix (two acyl chains of CL bind within the pocket while the other two keep inserted in the MIM) the Ups1-Mdm35 complex at MIM for further degradation.
 Appendix:
Appendix: