Mitochondria supply most of the adenosine triphosphate (ATP) in energy metabolism of the cells. The transport of ATP is mediated by two carriers, the AAC (ADP/ATP carrier) and the SCaMC (short Ca2+-binding mitochondrial carrier). These two carriers are homologs belonging to the mitochondrial carrier family, with about 30% sequence similarity. One major difference between SCaMC and AAC is that SCaMC can selectively transport MgATP while AAC cannot. Moreover, SCaMC has a higher selectivity for MgATP over ATP. However, the structural basis for the higher selectivity for MgATP remains elusive.
PhD candidate RUN Changqing and his colleagues from a research group led by Prof. CHOU James and OUYANG Bo at National Center for Protein Science Shanghai, Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, revealed the key structural difference between AAC and SCaMC, which explains SCaMC’s higher selectivity for transporting MgATP over ATP. In this study, the researchers first purified the transmembrane domain of SCaMC and performed a series of NMR experiments to achieve the backbone assignments. MnATP is used to probe the substrate binding sites due to its similarity to MgATP and its property of broadening the signal around it. Addition of increasing MgATP would specifically compete off MnATP in the binding sites and recover the signals. Using this method, researchers can calculate the apparent KD between MgATP and SCaMC. Proteoliposome assay showed that mutation of the aspartic acid residue (Asp361) dramatically reduced the selectivity for MgATP over ATP. The results indicate that Asp361 is a key residue responsible for the higher selectivity for MgATP over ATP.
This research entitled “Molecular Basis of MgATP Selectivity of the Mitochondrial SCaMC Carrier” has been published in Structure on July 9, 2015. This work was supported by grants from the Chinese Academy of Sciences, the National Natural Science Foundation of China, and NIH. The data were mainly collected at National Center for Protein Science Shanghai.
Contact:
CHOU James, Principal Investigator
National Center for Protein Science Shanghai, Institute of Biochemistry and Cell Biology, Shanghai Institutes of Biological Sciences, Chinese Academy of Sciences
Shanghai 201210, P. R. China
E-mail: jameschou@sibcb.ac.cn
OUYANG Bo, Principal Investigator
National Center for Protein Science Shanghai, Institute of Biochemistry and Cell Biology, Shanghai Institutes of Biological Sciences, Chinese Academy of Sciences
Shanghai 201210, P. R. China
E-mail: ouyang@sibcb.ac.cn
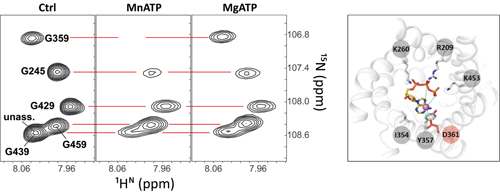
Figure: Illustration of the NMR method used to identify the binding sites of MgATP (left) and the model of how MgATP binds to SCaMC (right).
 Appendix:
Appendix: