Researchers Reveal the tRNA Recognition Mechanism of A Special SPOUT Methyltransferase TrmL
Source:
Time: 2015-09-20
The tRNAs play central roles in protein synthesis by transfer amino acids to ribosome. Nucleotides in tRNA are often post-transcriptionally modified, especially residues from the anti-codon region. Recently, researchers from Chinese Academy of Sciences reveal the tRNA recognition mechanism of a special RNA methyltransferase TrmL.
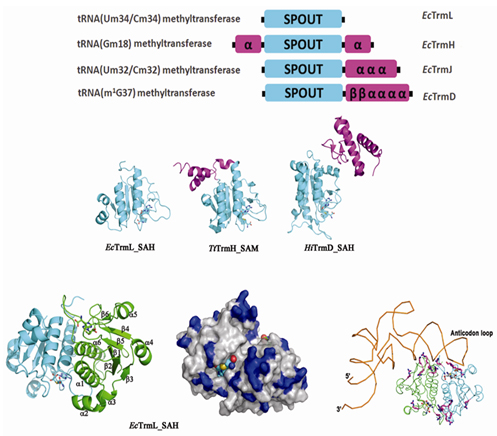
Modifications at wobble nucleotide are essential to the precise recognition of anti-codon on tRNA to codon on mRNA, and lack of these modifications can cause some severe cellular deficiency in vivo. TrmL is one of the latest identified tRNA modification enzymes reported in 2010, which is responsible for the 2’-O-methylation at position 34 in two Leucine tRNA isoacceptors (CAA and UAA). Unlike other tRNA-modifying enzymes from the SPOUT methyltransferase superfamily, the tRNA (Um34/Cm34) methyltransferase TrmL lacks the usual extension domain for tRNA binding, and consists only of a SPOUT domain. Both the catalytic and tRNA recognition mechanisms of this enzyme remain elusive.
Dr. LIU Ru-Juan, PhD student ZHOU Mi and their colleagues, led by Prof. WANG Enduo from Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences, identified the catalytic site and the tRNA initial binding mode of TrmL by solving the crystal structures of TrmL in apo form and in complex with S-adenosyl-homocysteine, site directed mutations, ITC and electrophoretic mobility shift assays.The results show that TrmL can independently catalyze the methyl transfer to two Leucine tRNA isoacceptors without the involvement of other tRNA binding proteins. Their discoveries suggest that TrmL functions as a homodimer by using the conserved C-terminal half of the SPOUT domain for catalysis, while residues from the less-conserved N-terminal half of the other subunit participate in tRNA recognition.
This study entitled “
The tRNA recognition mechanism of the minimalist SPOUT methyltransferase, TrmL” was published online in the
Nucleic Acids Research on June 26
th, 2013. It is supported by grants from the Ministry of Science and Technology of China, the National Natural Science Foundation of China, Chinese Academy of Sciences, and the Science and Technology Commission of Shanghai Municipality.
The minimalist protein TrmL from the SPOUT methyltransferase superfamily which lacks the usual tRNA binding domain functions as a homodimer to catalyze the ribose methylation of two Leu tRNA isoacceptors. (Image by Prof. WANG Enduo’s group)
 Appendix:
Appendix: