ECM1 (extracellular matrix protein 1 ) is an 85-kDa glycoprotein. Previous studies have been reported that it play a key role in malignancies, tumor progression, angiogenesis and skin physiology. However, the regulatory effect of ECM1 on immune system is unknown. It has been previously demonstrated that ECM1 is specifically secreted by Th2 (T helper type 2) cells and promotes their egress from draining lymph nodes in an animal model of asthma. However, its function during MOG-induced (myelin-oligodendrocyte-glycoprotein-induced) EAE (Experimental autoimmune encephalomyelitis) remains unknown.
Recently, a team of researchers, led by Prof. SUN Bing, at Institute of Biochemistry and Cell Biology (SIBCB), Shanghai Institutes for Biological Sciences (SIBS), Chinese Academy of Sciences(CAS), found a novel function of ECM1 in inhibiting Th17 (T helper type 17) differentiation in the EAE model, suggesting that ECM1 may have a potential to be used in clinical applications for understanding the pathogenesis of MS (Multiple sclerosis) and its diagnosis. This work was online published in The Journal of Immunology.
While Th17 cells are important for the disease induction, Th2 cells are inhibitory in this process. In the study, they reported the effect of a Th2 cell product, extracellular matrix protein 1 (ECM1), on the differentiation of Th17 cells and the development of experimental autoimmune encephalomyelitis (EAE). Their investigation observed that ECM1 administration from day 1 to day 7 following the EAE induction could ameliorate the Th17 cell responses and EAE development in vivo.
Further mechanism study revealed that ECM1 could interact with αv integrin on DC (dendritic cell) cells and suppress the αv integrin-mediated activation of latent TGF-β (transforming growth factor-beta), which is critical for activating latent TGF-β, resulting in an inhibition of Th17 differentiation at early stage of EAE induction. Furthermore, overexpression of ECM1 in vivo also significantly suppressed Th17 cell response and attenuated EAE induction in ECM1 transgenic mouse. Taken together, their study identified a novel function of ECM1 in inhibiting pathogenic Th17 cell development during EAE induction.
This study contributes to a deeper understanding of the pathogenesis of human multiple sclerosis (MS). In addition, the identification of ECM1 as a inhibitor of Th17 development may provide a potential therapeutic approach in Th17 cell-mediated diseases.
The study entitled “Novel Function of Extracellular Matrix Protein 1 in Suppressing Th17 Cell Development in Experimental Autoimmune Encephalomyelitis” has been published in The Journal of Immunology on 2016 Jun 17.
This work was conducted in collaboration with Prof. ZHU Jinfang’s lab from National Institute of Allergy and Infectious Diseases, and Prof. WU Zhiying’s lab from Zhejiang University School of Medicine.
This work was supported by grants from the National Natural Science Foundation of China (NSFC), National 973 key project (973 Program), Division of Intramural Research, NIAID, National Institutes of Health, USA and grant from NN-CAS foundation.
AUTHOR CONTACT:
Bing Sun. Ph.D. Professor
Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences
Biochemistry and Cell Biology Building, RM 1307
320 Yue Yang Road, Shanghai 200031, China
Tel: 86-21-54921376 Fax: 86-21-54921011
Email: bsun@sibs.ac.cn
KEYWORDS: ECM1, EAE, Immune regulation, TGF-b activation
NEWS ABSTRACT:
It has been known that Th17 cells play an important role in the induction and development of EAE. This study demonstrated that ECM1 could inhibit pathogenic Th17 cells generation and subsequently attenuated the development of MOG-induced EAE in mice through affecting αv integrin–mediated latent TGF-b activation in vivo and in vitro. The identification of ECM1 as a inhibitor of Th17 development may provide a potential therapeutic approach in Th17 cell-mediated diseases.
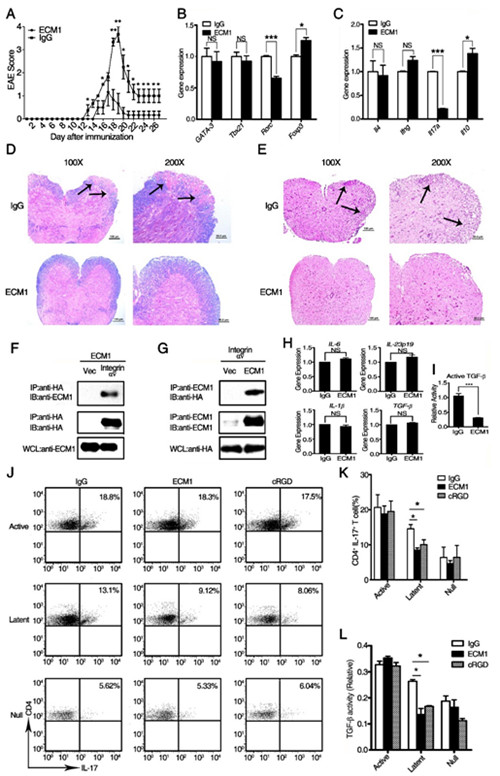
ECM1 attenuates MOG-induced EAE through inhibiting αv integrin-mediated TGF-b activation. (A): EAE clinical scores of ECM1 protein-treated mice (n=10) and human IgG control-treated mice (n=10). (B and C): The re-stimulated CD4+ T cells were isolated with MACS column from differentially treated EAE mice, then the transcription of T-bet, GATA-3, ROR-γt and Foxp3 (B) and the cytokines (C) IL-4, IFN-γ, IL-17 and IL-10 were evaluated using real-time PCR analysis. (D and E): Luxol fast blue (D) and H&E (E) staining of paraffin sections of spinal cords isolated from representative of human IgG control-treated and recombinant ECM1 protein-treated mice on day 21 after immunization. (F and G): ECM1 interacts with αv integrin. (H): The expression of IL-6, IL-23p19, IL-1β, and TGF-β in bone marrow-derived DCs(dendritic cells) cultured in the presence of recombinant ECM1 or human IgG proteins. (I): The activation of TGF-β in the supernatant of BMDCs(bone marrow dendritic cells) cultured in the presence of recombinant ECM1 or human IgG proteins. (J): The intracellular staining of IL-17 of the co-cultured T cells was investigated by flow cytometry. (K): The quantitative analysis of CD4+IL-17+ T cells in DC-T cell(dendritic cells-T cell)co-culture system. (L): The activity of active TGF-β in the supernatant of the co-culture system. (Image provided by Prof. SUN Bing’s lab)
 Appendix:
Appendix: