On Jan. 18th, 2017, Scientific Reports published online a work entitled “Molecular mechanism of the allosteric regulation of the ag heterodimer of human NAD-dependent isocitrate dehydrogenase” by Prof. Jianping Ding’s group at National Center for Protein Science Shanghai, State Key Laboratory of Molecular Biology, Center for Excellence in Molecular Cell Science, Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences. This work reveals the molecular basis of the allosteric regulation of the αγ heterodimer of human NAD-dependent isocitrate dehydrogenase.
Isocitrate dehydrogenases (IDHs) are a family of enzymes existing in all organisms, which catalyze the oxidative decarboxylation of isocitrate (ICT) into α-ketoglutarate (α-KG) using NADP or NAD as coenzyme. Eukaryotes contain both NADP-dependent IDHs (NADP-IDHs) and NAD-dependent IDHs (NAD-IDHs). NADP-IDHs, localized in the cytosol, mitochondria and peroxisomes, are demonstrated to play important roles in cellular defense against oxidative damage, detoxification of reactive oxygen species, and synthesis of fat and cholesterol. NAD-IDHs, localized in the mitochondria, exert the catalytic activity in the Krebs cycle.
In the past years, researchers from Prof. Ding’s group have determined the structures of several important eukaryotic NADP-IDHs, including human cytosolic and yeast mitochondrial NADP-IDHs (IDH1 and Idp1p) in complexes with substrate, coenzyme, metal ion or/and product, and the structure of an IDH1 mutant containing tumor-associated mutation R132H. These works reveal the structural basis for the biological function of NADP-IDHs and the pathogenesis of mutant IDH1 (J. Biol. Chem., 2004; Protein Sci., 2008; Cell Res., 2010). Mammalian NAD-IDHs are composed of three types of subunits in the ratio of 2α:1β:1γ, which can be activated by CIT and ADP through decreasing the Km for substrate without significantly affecting the Vmax. α subunit is essential to the catalysis of the enzyme, containing the binding sites for substrate, coenzyme and metal ion. β and γ subunits are the regulatory subunits, which are believed to have the ability to bind different regulators. It is also found that the α and β subunits and the α and γ subunits could be assembled to form the basic structural units of the holoenzyme, which exhibit some enzymatic activities. However, the structural and functional differences between two regulatory subunits of NAD-IDH and the molecular mechanism of allosteric regulation are largely unknown.
In this work, Dr. Tengfei Ma and his colleagues in Prof. Ding’s group solved the crystal structures of the αγ heterodimer with the γ subunit bound without or with the positive regulators CIT and ADP, and carried out detailed mutagenesis and kinetic studies to validate the functional roles of the key residues involved in the binding of the regulators and the structural communication between the allosteric and active sites. They found that CIT, ADP and Mg2+ bind adjacent to each other at the allosteric site in the γ subunit. The CIT binding induces significant conformational changes at the allosteric site, which are transmitted to the active site in the α subunit through the heterodimer interface, leading to stabilization of the ICT binding at the active site and thus activation of the enzyme. The ADP binding does not induce further conformational changes but enhances the CIT binding through Mg2+-mediated interactions, yielding a synergistic activation effect. Intriguingly, ICT can also bind to the CIT-binding site, which induces similar conformational changes but exhibits a weaker activation effect. The structural and biochemical data together reveal the molecular basis for the mechanism of the allosteric regulation of the αγ heterodimer, and provide new insights into the catalytic and regulatory mechanism of the human NAD-IDH holoenzyme.
Researchers would like to thank the staff members at BL19U1 of the National Facility for Protein Science in Shanghai (NFPSS) for technical supports in diffraction data collection. This study was supported by grants from the National Natural Science Foundation of China, the Ministry of Science and Technology of China and the Chinese Academy of Sciences.
AUTHOR CONTACT:
Jianping Ding, Ph.D., Professor
National Center for Protein Science Shanghai, State Key Laboratory of Molecular Biology, Center for Excellence in Molecular Cell Science, Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences
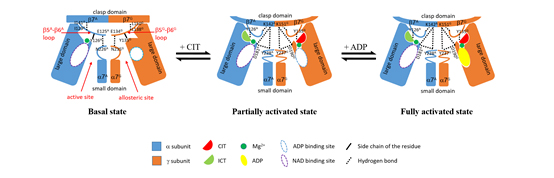
A schematic diagram showing the molecular mechanism of the allosteric regulation of the αγ heterodimer
 Appendix:
Appendix: