Researchers reveal substrate binding and catalytic mechanism of a human RNA:m5C methyltransferase NSun6
Source:
Time: 2017-05-24
5-methylcytosine is a common chemical modification in both DNA and RNA. m5C modifications have been extensively studied as important epigenetic markers in DNA. However, research on m5C modifications in RNA is relatively lagging behind. NSun methyltransferase family is the main RNA m5C modification enzymes in eukaryotes, which is composed of seven members. The genetic mutation of several NSun family members was reported to be closely related to many human diseases such as intellectual disability, male infertility and cancers. Due to lack of structural information, the RNA recognition mode and RNA:m5C modification mechanism of NSuns remain unclear for a long time. Meanwhile, the biological functions of RNA:m5C modification remain to be explored. In 2015, human NSun6 was identified as the methyltransferase catalyzing the m5C72 modification at the amino acid acceptor of many tRNAs. Last year, the report about the tRNA recognition elements by NSun6 revealed that NSun6 is a tRNA specific methyltransferse (Long et al, 2016, JBC).
Under the guidance of Prof. WANG Enduo, Dr. LIU Ru-Juan and Ph. D students LONG Tao et al. have revealed substrate binding and catalytic mechanism of hNsun6 through solving the crystal structures of hNSun6 in complex with tRNA, site directed mutations, ITC and electrophoretic mobility shift assays. They found that the PUA domain of hNSun6 recognizes both CCA end and D stem of tRNA, while the MTase domain of hNSun6 precisely recognizes C72 and U73. These recognition manners result in a non-canonical conformation of the bound tRNA, rendering the base moiety of the target cytosine accessible to the enzyme for methylation. Further biochemical assays verify the critical, but distinct, roles of two conserved cysteine residues for the RNA:m5C methylation.
This study entitled “Structural basis for substrate binding and catalytic mechanism of a human RNA:m5C methyltransferase NSun6” was published online in the Nucleic Acid Research on May 22, 2017. The work is supported by grants from the Ministry of Science and Technology of China, the National Natural Science Foundation of China, and Chinese Academy of Sciences.
CONTACT:
WANG En-Duo, Principal Investigator
Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences
Phone: 86-21-54921241
E-mail: edwang@sibcb.ac.cn
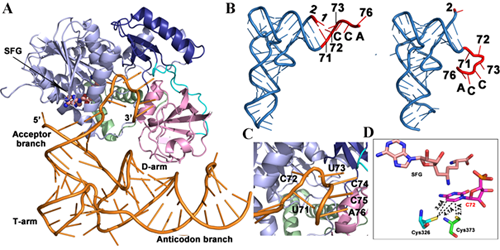
A. Overall structure of hNSun6 an tRNA complex; B. Structural comparison of tRNA (right) in the hNSun6 and tRNA complex compared to the typical structure of tRNA in free form (left); C. And D Insight into active site of hNSun6 (Image provided by Prof. WANG Enduo’s group)
Key Word: tRNA modification; m5C; methyltransferase; structure
https://academic.oup.com/nar/article/3844765/Structural-basis-for-substrate-binding-and
http://www.sibcb.ac.cn/ePI.asp?id=19

 Appendix:
Appendix: