A new joint work from Prof. En-Duo Wang’s lab, entitled “Editing activity for eliminating mischarged tRNAs is essential in mammalian mitochondria”, was online published by Nucleic Acids Research on Dec. 8, 2017.
Mammalian cells harbor two separate translation machineries, cytoplasmic and mitochondrial machineries. Human mitochondrial machinery produces 13 mitochondrial genome-encoded subunits of respiratory complexes, which are essential for the assembly of respiratory complexes and proper functions of mitochondria. A high level of fidelity is required by the cytoplasmic translation, which is mainly mediated by aminoacyl-tRNA synthetases (aaRSs), to ensure the accurate translation of the nuclear genetic code. For instance, abolishment or impairment of editing function of mouse cytoplasmic alanyl-tRNA synthetase (AlaRS) leads to accumulated unfolded/misfolded proteins, neuro-degeneration and cardioproteinopathy. However, the fidelity in mitochondrial genetic code flow and the in vivo significance of editing by mitochondrial aaRSs have been unknown.
In this work, we cloned, expressed human AARS2, encoding human mitochondrial AlaRS (hmtAlaRS) and purified hmtAlaRS. We showed that the hmtAlaRS mis-activated non-cognate Ser and was capable of editing mischarged Ser-tRNAAla in vitro; otherwise, mistranslation of mitochondria genome-encoded proteins would occur. Two mutants, hmtAlaRS-C749A and -V760E exhibited abolished or impaired post-transfer editing. Loss of the proofreading activity, due to mmtAlaRS C744A and V755E mutations, resulted in embryonic lethality in mice. These results indicated that tRNA proofreading is essential in mammalian mitochondria, and cannot be overcome by other quality control mechanisms. This is the first report about the in vivo significance of mammalian mitochondrial aaRSs.
Profs. Xiao-Long Zhou and En-Duo Wang are co-first and co-corresponding authors, respectively. This work is funded by the National Key Research and Development Program of China, the Natural Science Foundation of China, Chinese Academy of Sciences and the Committee of Science and Technology in Shanghai.
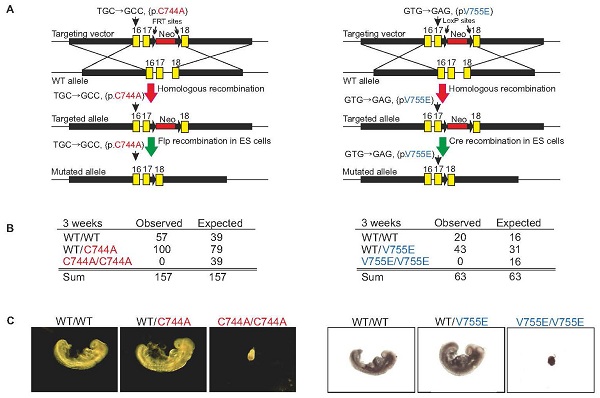
Figure. Editing-deficient knock-in mouse models of mtAlaRS are early embryonic lethal.
 Appendix:
Appendix: