Researchers find a novel aminoacyl-tRNA synthetase gene in mammalian cells
Source:
Time: 2018-03-21
Aminoacyl-tRNA synthetase (aaRS) catalyzes the esterification between amino acid and its cognate tRNA, to produce aminoacyl-tRNA for protein synthesis. With the evolution of organisms, aaRS has involved new functions beyond aminoacylation, such as angiogenesis, tumorigenesis, immune response, development and gene expression regulation, which are essential for cellular homeostasis. In general, the non-canonical functions of aaRSs are gained though protein-protein interaction mediated by their specific extensions.
Cytoplasmic and mitochondrial aaRSs in eukaryotes are usually encoded by two different nuclear genes. In human nuclear genome, there are 37 genes encoding all the aaRSs for the two organelles. Notably, in mammalian, besides TARS and TARS2 encoding cytoplasmic and mitochondrial threonyl-tRNA synthetases (ThrRSs), respectively, there exists an extra TARSL2 encoding a protein highly homologous to cytoplasmic ThrRS, which was named ThrRS-Like (ThrRS-L). The sequence of of ThrRS-L is conserved with ThrRS in the main body, but quite different in the N-terminal extension. The phylogenetic analysis revealed that, TARSL2 is derived from the duplication of TARS (Zhou et al., Nucleic Acids Res., 2013, 41, 302-314), representing the first duplicated aaRS gene in human. With conserved aminoacylation and editing domains and a mammalian-specific N-extension, whether ThrRS-L has canonical or non-canonical functions is an interesting scientific question.
Under the guidance of Profs. WANG En-Duo and ZHOU Xiao-Long, Ph.D students CHEN Yun et al. have studied the organ distribution pattern, cellular localization, canonical aminoacylation and editing activities of mouse ThrRS-L (mThrRS-L). Tarsl2 is ubiquitously but unevenly expressed in mouse tissues. Different from mouse cytoplasmic ThrRS (mThrRS), mThrRS-L can also locate in the nucleus via a nuclear localization sequence at its C-terminus. Based on the in vitro data, mThrRS-L was found to have similar aminoacylation and editing activities with mThrRS. However, mThrRS-L and mThrRS exhibit differences in tRNA recognition and editing capacity.
This study entitled “A threonyl-tRNA synthetase-like protein has tRNA aminoacylation and editing activities” was published online in the Nucleic Acid Research on March 20, 2018. The work is supported by grants from Strategic Priority Research Program and Youth Innovation Promotion Association of the Chinese Academy of Sciences, National Key Research and Development Program and Natural Science Foundation of China, Committee of Science and Technology in Shanghai, Shanghai Rising-Star Program and China Postdoctoral Science Foundation.
CONTACT:
WANG En-Duo, Principal Investigator
State Key Laboratory of Molecular Biology, CAS Center for Excellence in Molecular Cell Science, Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences
Shanghai 200031, P. R. China
Phone: 86-21-54921241
E-mail: edwang@sibcb.ac.cn
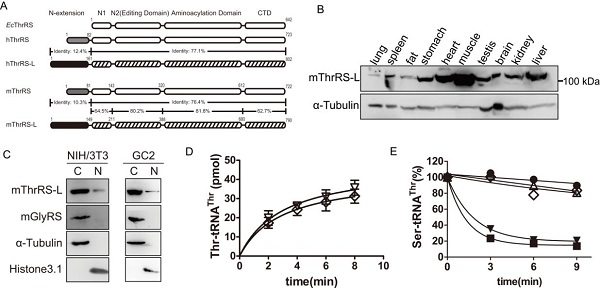
The distribution pattern, localization, enzymatic activities of mThrRS-L

 Appendix:
Appendix: