Nerve Injury-Induced Neuronal PAP-I Maintains Neuropathic Pain by Activating Spinal Microglia
Source:
Time: 2020-01-15
Neuropathic pain is maladaptive pain condition and the maintaining mechanism is largely unclear. Pancreatitis-associated proteins (PAPs), the secretory proteins, belong to the calcium-dependent lectin gene superfamily. PAPs have been shown to play roles in the digestive system, while to engage in neural regeneration after nerve injury. However, whether PAPs participate in pain development is still unclear.
A recent study ‘‘Nerve Injury-Induced Neuronal PAP-I Maintains Neuropathic Pain by Activating Spinal Microglia’’ was published in The Journal of Neuroscience on January 8, 2020. This work was performed by researchers in Dr. ZHANG Xu’s Lab at Institute of Neuroscience and State Key Laboratory of Neuroscience, Center for Excellence in Brain Science and Intelligence Technology, and in Dr. BAO Lan’s Lab at State Key Laboratory of Cell Biology, Center for Excellence in Molecular Cell Biology / Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences.
To address the question, researchers constructed the rat spared nerve injure (SNI) model and found that peripheral nerve injury triggers the
de novo expression of PAP-I only in dorsal root ganglia (DRG) neurons, the sizes of which shift from small to large and back to small ones, indicating the potential role of PAP-I in neuropathic pain. The increased PAP-I was then transported bi-directionally toward peripheral and central terminals. To distinguish the function of PAP-I in two terminals, the researchers performed intrathecal injection and intraplantar injection of PAP-I respectively to rats and observed the pain behavior. The results showed that intrathecal but not intraplantar application of PAP-I induced thermal and mechanical hyperalgesia, indicating that PAP-I is pro-nociceptive in the spinal cord. In contrast, behavior test in PAP-I knockout rats with SNI surgery developed tactile allodynia within the first week, but tactile allodynia was alleviated significantly at Day 43, which means PAP-I is not necessary in the initiation but a major factor for long-term maintenance of tactile allodynia. To further explain the involved mechanisms, the researchers found the activation of microglia in spinal dorsal horn after
PAP-I injection. Additional experiments confirmed PAP-I-CCR2-p38 MAPK pathway in microglia activation. Finally, an inhibition of microglia activation or CCR2 reversed PAP-I-induced thermal and mechanical hyperalgesia. Taken together, PAP-I mediates the neuron-microglial crosstalk after peripheral nerve injury and contributes to the maintenance of neuropathic pain.
This work was funded by the National Natural Science Foundation of China, the strategic priority research program (B) of Chinese Academy of Sciences, and the key research program of frontier sciences, Chinese Academy of Sciences, and the Science and Technology Commission of Shanghai Municipality, China. This work was also collaborated with Dr. CHEN Luonan’s Lab at Center for Excellence in Molecular Cell Biology / Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences.
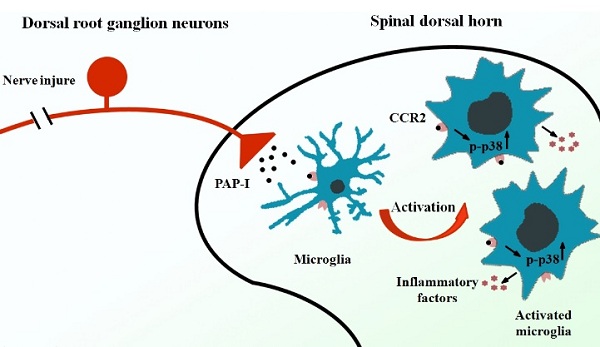
Figure 1 PAP-I is induced in DRG neurons after peripheral nerve injury and transported to spinal dorsal horn, and then activates microglia via CCR2-p38 MAPK pathway, involving in the maintenance of neuropathic pain.
Contact: baolan@sibcb.ac.cn

 Appendix:
Appendix: