In a study published in Journal of Cell Biology, the research team led by Prof. ZHU Xueliang from the Center for Excellence in Molecular Cell Science, Shanghai Institute of Biochemistry and Cell Biology of the Chinese Academy of Sciences, collaborated with Prof. YAN Xiumin from Xinhua Hospital affiliated with Shanghai Jiao Tong University School of Medicine and Prof. LI Dong from the Institute of Biophysics, Chinese Academy of Sciences, revealed the functions of the kinesin proteins Kif6 and Kif9 in mammalian motile cilia.
Motile cilia and flagella are evolutionarily conserved, hair-like organelles extending from the cell surface, typically comprising nine doublet microtubules (DMTs) and a central pair of MT singlets. Motile cilia are commonly found in multicilia and are widely distributed in the walls of mammalian brain ventricles, trachea, oviducts, and efferent ducts. Their coordinated directional beats across entire tissues drive directional cerebrospinal fluid (CSF) flow, mucous clearance, and ovum transport while flagella are responsible for the movement of sperm. Kinesins, one of the crucial molecular motors, capable of taking nanometer steps along the MTs, are involved in a variety of biological processes including cargo transport, cell division, and ciliogenesis. They also power the trains that are responsible for intraflagellar transport (IFT), the process by which cargos are transported in and out of cilia and flagella.
The kinesin-9 family consists of two members, Kif6 and Kif9, that share a high sequence identity (Fig. A). Phylogenetic analysis showed that Kif6 and Kif9 co-existed in eukaryotes with motile cilia/flagella, but their function in mammals remains unclear. The team employs a self-built high-speed and super-resolution grazing incidence structured microscopy (GI-SIM) and discovered that Kif6 and Kif9 have distinct localizations and behaviors in mammalian motile cilia. Kif6 forms as puncta that move bidirectionally along the DMTs, with some co-migrating with IFT trains, whereas Kif9 puncta did not exhibit apparent long-distance movements along axonemes (Fig. B-C). Consistently, only Kif6 but not Kif9 displays microtubule-based motor activity in vitro.
Kif6 deficiency in mice disrupts coordinated ciliary beat across ependymal tissues and impairs cerebrospinal fluid flow, resulting in severe hydrocephalus and high mortality. Kif9 deficiency causes mild hydrocephalus without obviously affecting the ciliary beat or the lifespan (Fig. D-E). Kif6-/- and Kif9-/- males are infertile but exhibit oligozoospermia with poor sperm motility and defective forward motion of sperms, respectively. These results suggest Kif6 appears to aid cargo transport along DMTs to facilitate coordinated directional beats of ependymal multicilia, whereas Kif9 as a regulator fine-tunes ciliary beat on the central apparatus (Fig. F). This study provides new insights into the molecular regulatory mechanisms of ciliary motility and related pathological mechanisms.
Reference: https://doi.org/10.1083/jcb.202312060
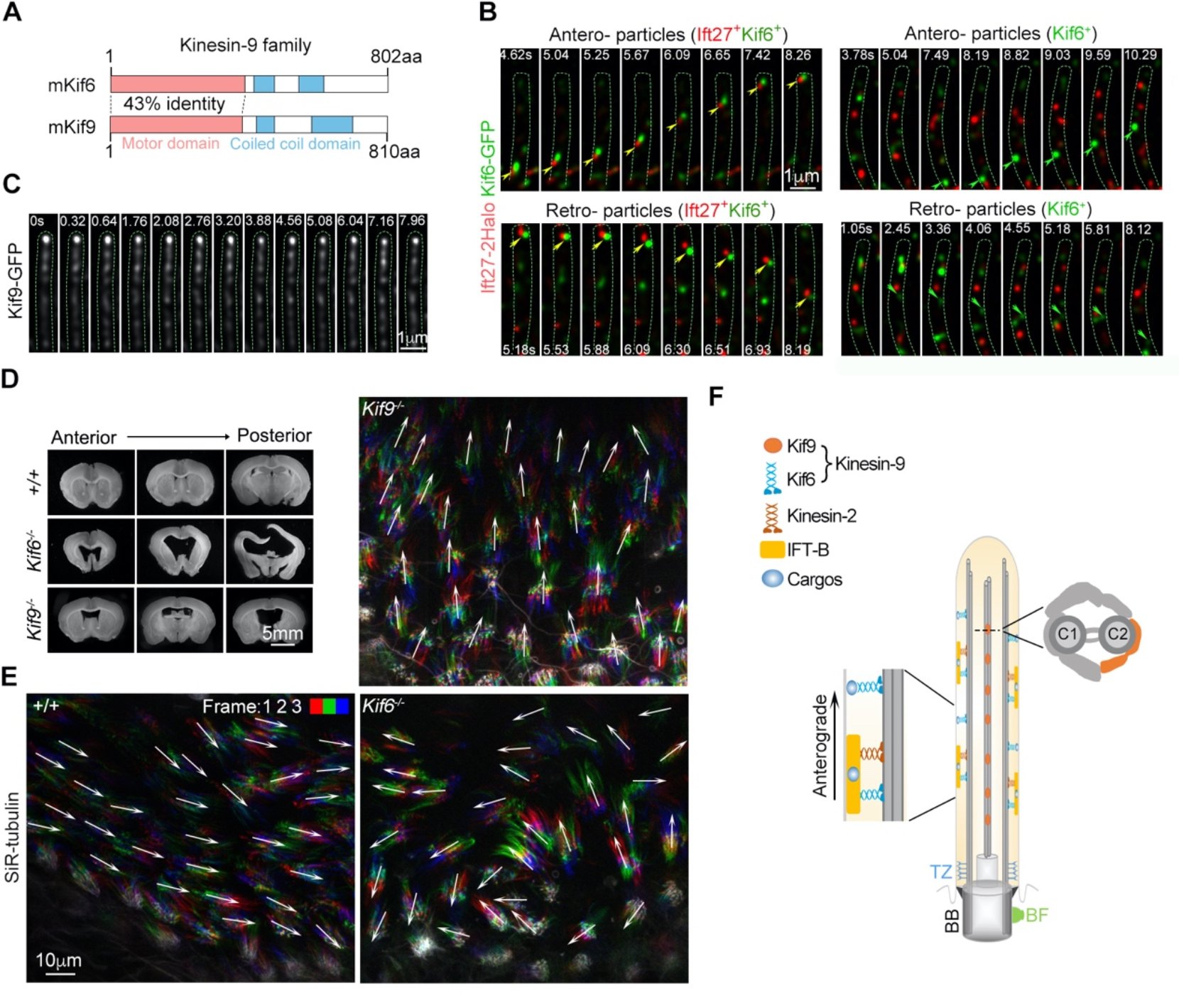
(A)Schematics of full-length murine Kif6 and Kif9. (B) Kif6-GFP undergoes long-range bidirectional movements along DMTs of ependymal cilia, with some co-migrating with Ift27 particles (Ift27+Kif6+) and others moving independently (Kif6+). (C)Kif9-GFP exhibits short-range oscillating movements in the central apparatus; (D) Coronal brain slices for ventricle expansions reveal severe hydrocephalus in Kif6-deficient (Kif6-/-) mice, while Kif9-deficient (Kif9-/-) mice show only mild hydrocephalus; (E) Unlike wild-type (+/+) and Kif9-deficient mice, Kif6-deficient mice exhibit varied ciliary beat directions across ependymal tissues; (F) Working model of Kif6 and Kif9 in motile cilia.
 Appendix:
Appendix: